
In 2009, LLS TAP invested $4.1 million in a phase 2 trial of CPX-351. After promising results, LLS TAP invested another $5 million into a phase 3 trial. The FDA granted Breakthrough Therapy designation in 2016, and CPX-351 was approved a year later for a number of blood cancers—including the first FDA approvals for two types of adult AML. After Celator’s acquisition by Jazz Pharmaceuticals LLS saw a return of $25.3 million.
Click here to view a larger image of the TAP-Celator Success Story!
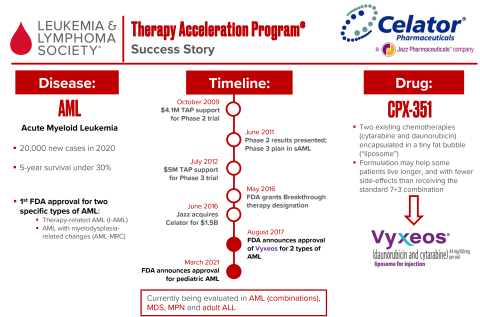
Vyxeos® (CPX-351), an innovative reformulation of two chemotherapies, was approved by the U.S. Food and Drug Administration on August 3 2017 and is the first approved treatment for adult patients with certain types of high-risk acute myeloid leukemia (AML): newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC). On March 30 2021, the FDA revised the approval label to include pediatric patients aged 1 year and older.
Clinical data that supported the first approval application was published in J Clin Oncol.

