Publication of CPX-351 Clinical Data in 'Blood Advances': Phase 3 Post-Hoc Analyses
Former TAP Partner

CPX-351 (Vyxeos, daunorubicin and cytarabine liposome for injection) is a dual-drug liposomal encapsulation of daunorubicin and cytarabine approved by the US Food and Drug Administration in 2017 and the European Medicines Agency in 2018 for the treatment of adults with newly diagnosed t-AML or AML with myelodysplasia-related changes (AML-MRC). CPX-351 was designed to improve efficacy versus conventional chemotherapy through the coencapsulation of daunorubicin and cytarabine at a synergistic 1:5 molar ratio. In the primary analysis of data from the pivotal randomized phase 3 study in older adults with newly diagnosed high-risk/sAML, induction followed by consolidation with CPX-351 demonstrated a significantly improved median overall survival (OS [primary endpoint]; 9.56 vs 5.95 months) and a higher rate of complete remission (CR) or CR with incomplete neutrophil or platelet recovery (CRi) vs conventional 7+3 (48% vs 33%), with a safety profile that was consistent with the known safety profile of 7+3. After 5 years of follow-up, survival benefit was maintained.
Further analyses were recently published in an article entitled "Older adults with newly diagnosed high-risk/secondary AML who achieved remission with CPX-351: phase 3 post hoc analyses" in the journal Blood Advances.
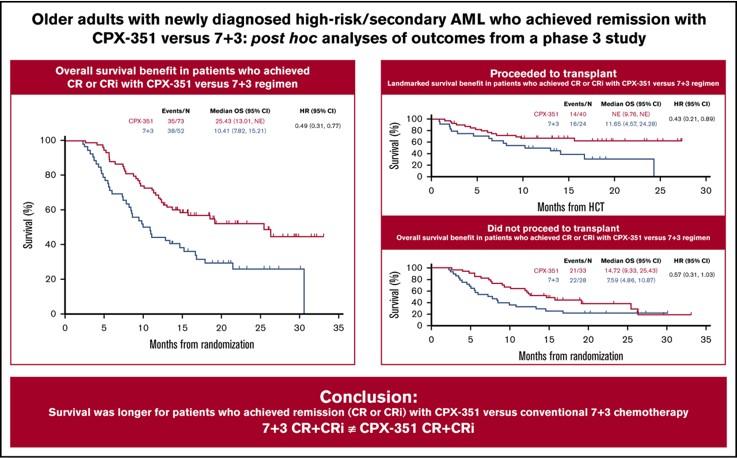
Exploratory post hoc subgroup analyses were evaluated to determine the impact of achieving complete remission (CR) or CR with incomplete neutrophil or platelet recovery (CRi) with CPX-351 (73/153 [48%]) vs conventional 7+3 (52/56 [33%]) on outcomes. CPX-351 improved median OS vs 7+3 in patients who achieved CR or CRi (25.43 vs 10.41 months). Improved median OS was seen across AML subtypes (t-AML, AML-MRC), age subgroups (60 to 69 vs 70 to 75 years), patients with prior hypomethylating agent exposure, and patients who did not undergo transplantation. Patients who achieved CR or CRi with CPX-351 also had a higher rate of transplantation, a longer median OS landmarked from the date of transplantation (not reached vs 11.65 months), and a safety profile that was consistent with the known safety profile of 7+3. These results suggest deeper remissions may be achieved with CPX-351, leading to improved OS.
LLS funded both Phase 2 and Phase 3 clinical development of Vyxeos through its Therapy Acceleration Program® (TAP) by partnering and supporting Celator Pharmaceuticals between in 2009-2016. Celator was acquired by Jazz Pharmaceuticals in 2016. Under the TAP venture philanthropy initiative, LLS partners directly with biotechnology companies to accelerate the development of promising therapies for unmet medical needs, many of which otherwise might not receive support.