On this page:
- Introduction
- Chimeric Antigen Receptor T-Cell Therapy: How it Works
- FDA Approved Treatments
- Dr. Brentjens Talks about CAR T-Cell Therapy
- Possible Side Effects of CAR T-Cell Therapy
- Results, Limitations, and the Future of CAR T-Cell Therapy
- Video about CAR T-Cell Therapy
- Enrolling in a Trial
- Additional Resources
- Sponsors & Supporters
Introduction
The immune system is the body’s defense against infection and cancer. It is made up of billions of cells that are divided into several different types.
Lymphocytes, a subtype of white blood cells, comprise a major portion of the immune system. There are three types of lymphocytes
- B lymphocytes (B cells) make antibodies to fight infection.
- T lymphocytes (T cells) have several functions, including helping B lymphocytes to make antibodies to fight infection, and directly killing infected cells in the body.
- Natural killer cells also attack infected cells and eliminate viruses.
Immunotherapy
- Is a type of treatment that utilizes the body’s own immune system to fight cancer
- Improves the body’s ability to detect and kill cancer cells
- Is based on the concept that immune cells or antibodies can recognize and kill cancer cells.
Immune cells or antibodies can be produced in the laboratory under tightly controlled conditions and then given to patients to treat cancer. Several types of immunotherapy are either approved for use or are under study in clinical trials to determine their effectiveness in treating various types of cancer.
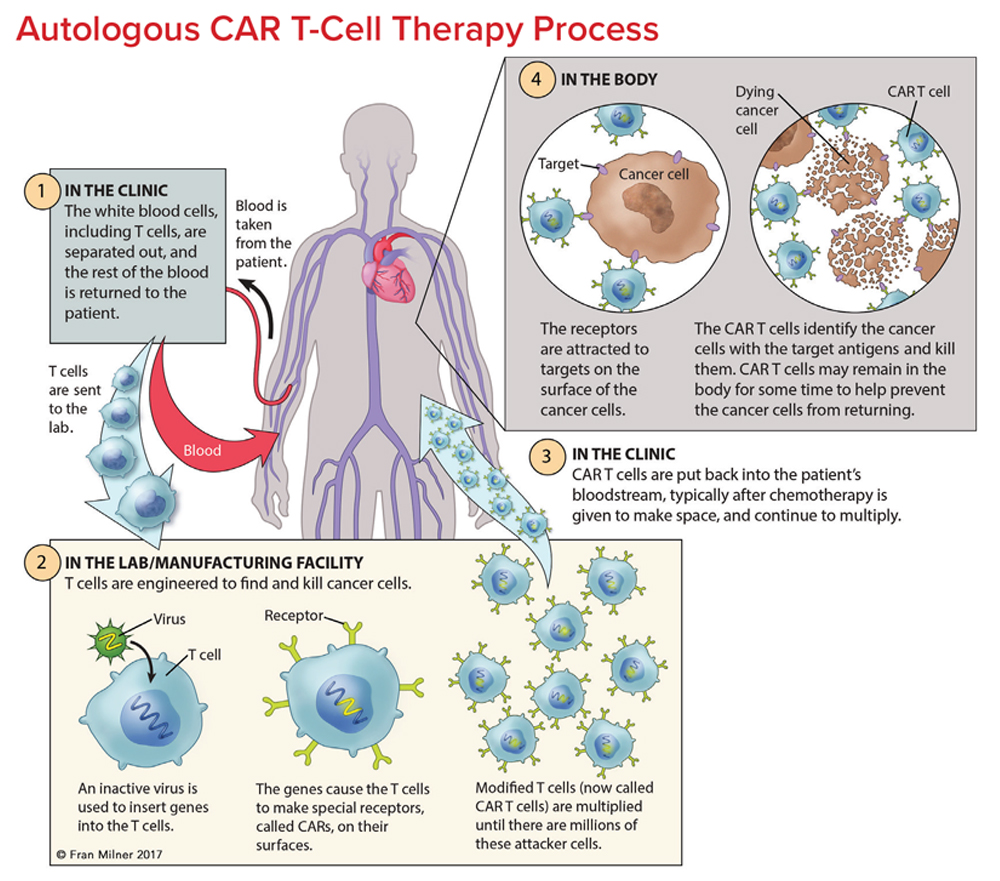
T cells are collected from a patient. T cells are collected via apheresis, a procedure during which blood is withdrawn from the body and one or more blood components (such as plasma, platelets or white blood cells) are removed. The remaining blood is then returned to the body.
T cells are reengineered in a laboratory. The T cells are sent to a laboratory or a drug manufacturing facility where they are genetically engineered, by introducing DNA into them, to produce chimeric antigen receptors (CARs) on the surface of the cells.
After this reengineering, the T cells are known as “chimeric antigen receptor (CAR) T cells.” CARs are proteins that allow the T cells to recognize an antigen on targeted tumor cells.
The reengineered CAR T cells are then multiplied. The number of the patient’s genetically modified T cells is “expanded” by growing cells in the laboratory. This takes about 3 to 4 weeks. When there are enough of them, these CAR T cells are frozen and sent to the hospital or center where the patient is being treated.
At the hospital or treatment center, the CAR T cells are thawed and then infused into the patient. Many patients are given a brief course of one or more chemotherapy drugs to reduce the number of normal t cells in the body before they receive the infusion of CAR T cells. This is called “lymphodepletion,” and it makes space for the new CAR T cells. The new CAR T cells are infused into the patient’s bloodstream by IV or through an existing central line. This process takes less than 30 minutes. The CAR T cells that have been returned to the patient’s bloodstream multiply in number. These are the “attacker” cells that will recognize, attack and kill cells that have the target antigen on their surface.
The CAR T cells may help guard against recurrence. CAR T cells may not only eradicate all cancer cells in the body, but they may remain in the body months after the infusion. The therapy has resulted in long-term remissions for some patients with certain types of blood cancer.
FDA Approved Treatments
- Axicabtagene ciloleucel (Yescarta™)
- Brexucabtagene autoleucel (Tecartus®)
- Ciltacabtagene autoleucel (Carvykti™)
- Idecabtagene vicleucel (Abecma®)
- Lisocabtagene maraleucel (Breyanzi®)
- Obecabtagene autoleucel (Aucatzyl®)
- Tisagenlecleucel (Kymriah®)
- Tocilizumab (Actemra®)
CAR T-cell therapy continues to be available to patients who are participating in a clinical trial for indications or cancers other than the FDA approved indications. Trial protocols vary. Depending on the clinical trial, care may be provided in either a hospital setting or an intensive outpatient treatment center with experience administering cellular immunotherapy. Patients may have to stay at the treatment facility and may need to plan to stay close by before, during or following treatment. Some trial protocols require patients to confirm the availability of a caregiver before they can enroll in the trial.
Possible Side Effects of CAR T-Cell Therapy
While many patients have reported only mild to moderate side effects with CAR T-cell therapy, this treatment is sometimes associated with serious side effects. It is important to speak with the healthcare team about potential side effects before starting any treatment. Most side effects resulting from CAR T-cell therapy will either resolve on their own or can be managed with appropriate treatment.
Cytokine-Release Syndrome (CRS). This potentially serious side effect is frequently associated with CAR T-cell therapy. Cytokines (chemical messengers that help the T cells carry out their functions) are produced when the CAR T cells multiple in the body and kill the cancer cells.
When the CAR T cells encounter their antigen targets, they are rapidly activated. At this point, numerous inflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNFα) and interferon gamma (IFNγ), are released. The large amounts of cytokines produced and then released by the activated immune system cause a collection of mild to potentially life-threatening signs and symptoms.
Common symptoms of CRS include:
- Fever
- Fatigue
- Headache
- Hypotension (low blood pressure)
- Hypoxia (lack of oxygen reaching the tissue)
- Tachycardia (abnormally rapid heart rate)
- Chills
The symptoms of CRS can also be more serious such as:
- Capillary leakage (fluid and proteins leaking out of tiny blood vessels and flowing into surrounding tissues, resulting in dangerously low blood pressure and difficulty breathing)
- Cardiac arrest (the heart stopping)
- Cardiac arrhythmias (abnormal heartbeat)
- Cardiac failure
- Hemophagocytic lymphohistiocytosis (HLH) (life-threatening immune system condition when T and NK cells become overactive causing too much inflammation)/macrophage activation syndrome (MAS) (an uncontrolled immune system working overtime, leading to inflammation)
- Renal insufficiency (poor kidney function)
- Poor lung oxygenation
- Multiple organ failure
Severe CRS requires intensive care treatment. Although most symptoms are reversible, the potential life-threatening risk of CAR T-cell therapy should not be underestimated. Deaths have been reported in CAR-T cell therapy trials.
Depending on the patient and the CAR T cells, CRS may occur within 1 to 21 days of CAR T-cell infusion. The duration of CRS is variable and it depends on the type of intervention used to manage it.
Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). Common signs and symptoms of ICANS include:
- Language impairment (aphasia)
- Confusion
- Delirium
- Involuntary muscle twitching
- Hallucinations
- Unresponsiveness
- Seizures
The connection between CRS and neurologic adverse events is not completely understood. The frequency, severity and nature of neurological toxicity is different among CAR T-cell products. The underlying cause of ICANS is unclear. The cause of neurotoxicity is the subject of intense investigation by researchers.
Neurotoxicity is reversible in most cases, and signs and/ or symptoms usually resolve over several days without intervention or apparent long-term effects. However, neurologic complications of CAR T-cell therapy can be life-threatening. Harmful neurological events have been reported. Cerebral edema (swelling in the brain) is the most common. Additionally, there have been fatalities. Some symptoms of neurologic toxicity can be treated with anti-epileptic medication and/or corticosteroids. Some patients may receive prophylactic (preventative, before CAR T-cell therapy) anti-epileptic medications. Sometimes a lumbar puncture (a procedure typically used to remove a sample of spinal fluid for testing) may be used to relieve pressure from brain swelling caused by severe ICANS.
Signs and symptoms of ICANS can sometimes be subtle. As a result, patients are frequently asked to complete a series of assessments during their treatment to ensure that they do not have neurologic toxicities. This assessment may include asking patients to write a sentence, to report the date, or perform other simple tasks to demonstrate that they do not have any neurologic symptoms.
Tumor Lysis Syndrome (TLS). Another known side effect of CAR T-cell therapy is tumor lysis syndrome (TLS), a group of metabolic complications that can occur due to the breakdown of dying cells—usually at the onset of toxic cancer treatments. However, TLS can be delayed and may occur one month or more after CAR T-cell therapy. TLS can cause organ damage and can be a life-threatening complication of any treatment that causes breakdown of cancer cells, including CAR T cells. The complication has been managed by standard supportive therapy.
Anaphylaxis (Life-threatening Allergic Reaction). There is potential for a patient receiving CAR T-cell therapy to have an overwhelming immune response against the CAR itself, called “anaphylaxis.” Symptoms associated with anaphylaxis include hives, facial swelling, low blood pressure and respiratory distress. There have been a few reports of acute anaphylaxis. Thorough monitoring and immediate treatment of this life-threatening side effect are critical for patients receiving CAR T-cell therapy.
B-Cell Aplasia. CAR T-cell therapy targeting antigens found on the surface of B cells not only destroys cancerous B cells but also normal B cells. Therefore, B-cell aplasia (low numbers of B cells or absent B cells) is an expected result of successful CD19-specific CAR T-cell treatment and has served as a useful indicator of ongoing CAR T-cell activity. This effect results in less ability to make the antibodies that protect against infection. Intravenous or subcutaneous immunoglobulin replacement therapy may be given to prevent infection. Long-term follow-up study is needed to assess the late effects of B-cell aplasia.
Infection. A number of patients (20% to 40%) who receive CAR T-cell therapy may have prolonged low blood cell counts. Low white blood cell count can result in serious bacterial, viral or fungal infections. Additionally, opportunistic infections (infections that occur due to a unique opportunity, such as a weakened immune system) can occur. The most common types of infections occur within the first three months following the CAR T-cell infusion are upper and lower respiratory tract infections.
As a precautionary measure, following CAR T-cell therapy, depending on the patient’s blood cell count recovery, most patients will be maintained on prophylactic antimicrobial therapy (treatment designed to prevent an infection from occurring).
Results, Limitations, and the Future of CAR T-Cell Therapy
CAR T-cell clinical trials have generated impressive results in the early outcomes of CAR T-cell therapy patients with blood cancers.
Chimeric antigen receptor T-cell clinical trials have generated impressive results in the early outcomes of patients with blood cancers. With the FDA approvals multiple drugs, CAR T-cell therapy represents a potential to treat certain leukemias, lymphomas and myeloma in patients whose disease has relapsed or is refractory to treatment.
In some studies, up to 90 percent of children and adults with B-ALL, whose disease had either relapsed multiple times or failed to respond to standard therapies, achieved remission after receiving CAR T-cell therapy. Even though some of these therapies have only been recently approved by the FDA, they have been studied for many years in clinical trials prior to their approval. Data from long-term outcome studies following CAR T-cell therapy indicates that CD19-targeted CAR T cells can induce prolonged remissions in patients with B cell malignancies, while remissions induced by BCMA-targeted CAR T cells are typically more short-lived. Additionally, certain patient and disease factors are associated with achieving durable remissions after CAR T-cell therapy.
While CAR T-cell therapy has achieved great clinical results, there are some disadvantages to this type of therapy. The products are generated from a patient’s autologous T cells, which requires extensive and costly collection and manufacturing efforts. The time between apheresis (when the patient’s T cells are collected) to the infusion of the engineered CAR T cells back to the patient is called the “vein-to-vein” time. Currently, all FDA-approved products require three to five weeks of manufacturing and quality assessment before the product is available to the patients. This delay can be problematic in some patients with certain diseases, such as acute leukemia, who may show disease progression before an autologous CAR T-cell treatment is ready for use.
Researchers have started to rethink the source of immune cells to produce CAR T-cell therapies to address some of the current limitations of this type of therapy. Using T cells collected from healthy donors or using umbilical cord blood are approaches used to produce “off-the-shelf” allogeneic CAR T cells.
The use of allogeneic CAR T cells has many potential advantages. These include decreased costs. The reduction in costs is due to the implementation of industrialized processes, which produce a large number of CAR T cells that can be produced from a single donor and become immediately available for treatment in cancer patients.
This approach is being pursued by several manufacturing companies and is under study in clinical trials for hematological malignancies, including B-cell ALL, AML, NHL and myeloma.
Video about CAR T-Cell Therapy
Enrolling in a Trial
Many CAR-T cell therapies are being studied in clinical trials for indications or cancers other than the FDA approved indications. Talk with your doctor about whether participation in a CAR T-cell therapy clinical trial is an option for you. Obtaining another opinion from a hematologist-oncologist (a blood cancer specialist), may be helpful in finding additional clinical-trial information as well. When you and your doctor discuss CAR T-cell therapy as a potential treatment option for you, it may be helpful to have
- A list of questions to ask concerning risks versus benefits of such a trial (click here for lists of suggested questions).
- A family member, friend, or another advocate with you for support and to take notes.
Clinical Trial Support Center
Work one-on-one with an LLS Clinical Trial Nurse Navigator who will help you find clinical trials and personally assist you throughout the entire clinical-trial process.
For more information or to contact us, click here.
Additional Resources
For Patients
- Contact an LLS Information Specialist for more information
- Download or order these free materials:
- Webcasts
- Education Videos
- Podcast Episodes
For Healthcare Professionals
- Fact sheet (Available to order or download)
- Podcast episodes
- CAR T-cell and Bispecific Therapies Webinar

